Chemical Ligation and Isotope Labeling to Locate Dynamic Effects during Catalysis by Dihydrofolate Reductase
Abstract
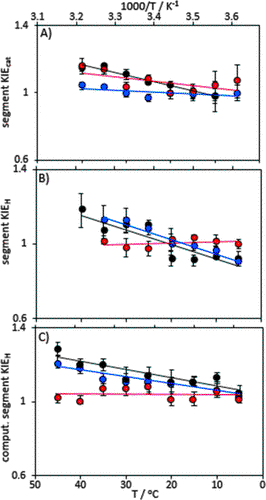
In this work, we investigated the effects of localized motional changes on enzyme catalysis by using chemical ligation to alter motions in specific regions of dihydrofolate reductase from E. coli. We prepared two isotopic hybrids: one with the mobile N-terminal segment containing heavy isotopes (²H, ¹³C, ¹⁵N) and the remainder of the protein with natural isotopic abundance, and the other with only the C-terminal segment isotopically labeled. Through kinetic investigations, we demonstrated that isotopic substitution of the N-terminal segment affected only a physical step of catalysis, while the enzyme chemistry was influenced by protein motions originating from the C-terminal segment. QM/MM studies supported the conclusion that dynamic effects on catalysis primarily arise from the C-terminal segment. We showed that the use of isotope hybrids provides unique insights into the microscopic mechanism of dynamic coupling, which are difficult to obtain through other methods, and helps define the dynamic networks of intramolecular interactions that are central to enzyme catalysis.