Isotope Substitution of Promiscuous Alcohol Dehydrogenase Reveals the Origin of Substrate Preference in the Transition State
Abstract
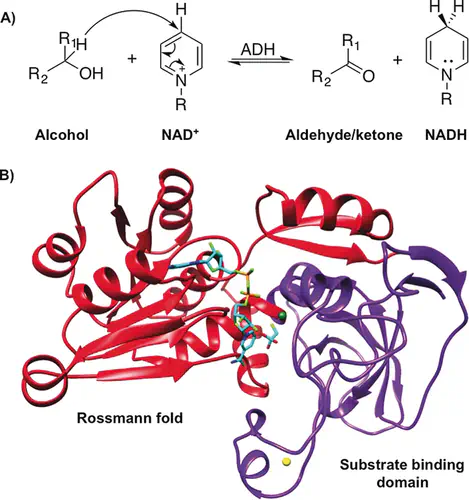
In this work, we studied the origin of substrate preference in promiscuous enzymes by studying the alcohol dehydrogenase from Geobacillus stearothermophilus (BsADH) through enzyme isotope labeling. We found that, at physiological temperature, protein dynamic coupling to the reaction coordinate was negligible. However, at lower temperatures, the extent of dynamic coupling became highly dependent on the substrate. For benzyl alcohol, we observed an enzyme isotope effect larger than unity, while for isopropanol, the enzyme isotope effect was close to unity. Frequency motion analysis of the transition states revealed that residues surrounding the active site undergo significant displacement during catalysis when sterically bulky alcohols are involved. Our results indicate that BsADH prefers smaller substrates, which generate less protein friction along the reaction coordinate and reduce the frequency of dynamic recrossing. Based on these findings, we proposed a hypothesis that predicts the trend of enzyme isotope effects for a wide range of substrates.