Temperature dependence of dynamic, tunnelling and kinetic isotope effects in formate dehydrogenase
Abstract
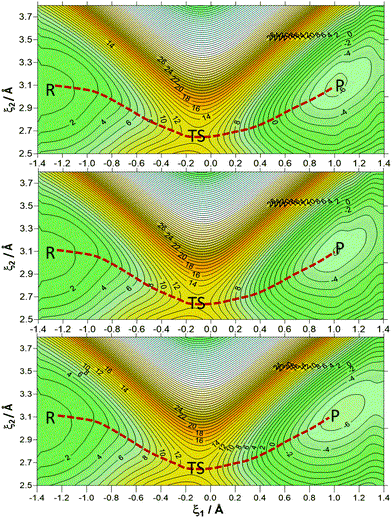
Here we explored the origin of the catalytic power of enzymes, focusing on the controversial role of protein dynamics in enzymatic catalysis. Specifically, we studied the hydride transfer step in the formate dehydrogenase (FDH EC 1.2.1.2) enzyme using molecular dynamics (MD) simulations with quantum mechanics/molecular mechanics (QM/MM) potentials. Our goal was to investigate potential correlations between protein dynamics, tunneling effects, and the rate constant. We computed the temperature dependence of kinetic isotope effects (KIEs), a key experimental and computational test to address this debate, and compared our results with previous experimental data. We found that the classical mechanical free energy barrier and the number of recrossing trajectories is temperature-independent, while quantum vibrational corrections and tunneling effects exhibit a slight temperature dependence within the range of 5 - 45 °C. The computed primary KIEs showed excellent agreement with experimental data and were nearly temperature-independent within standard deviations, with the modest temperature dependence attributed solely to quantum vibrational corrections. Additionally, we analyzed collective variables such as the electrostatic potential and the electric field created by the protein on key atoms involved in the reaction. These results confirmed that while the protein is well preorganized, specific changes occur along the reaction that facilitate hydride transfer and product release. These changes are defined by coordinates that form part of the real reaction coordinate.