Loss of Hyperconjugative Effects Drives Hydride Transfer during Dihydrofolate Reductase Catalysis
Abstract
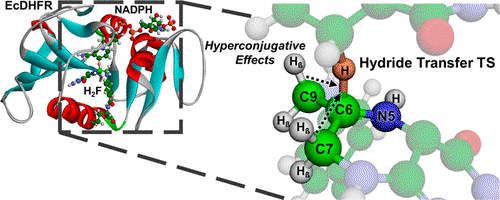
In this paper, we studied hydride transfer, a widespread process in nature with significant importance in applied research, focusing on the mechanisms by which this transformation occurs in living organisms. Using dihydrofolate reductase (DHFR), an enzyme that catalyzes the transfer of a hydride from C4′ of NADPH to C6 of 7,8-dihydrofolate (H2F), we investigated the role of polarization of the PI-bond of H2F in driving hydride transfer. To address this, we stereospecifically labeled H2F with deuterium β to the reacting center and measured β-deuterium kinetic isotope effects. Combining experimental results with analysis from QM/MM simulations, we demonstrated that hydride transfer is triggered by polarization at C6 of H2F. We also found that the σ Cβ-H bonds contribute to the buildup of cationic character during the chemical transformation, and hyperconjugation influences the formation of the transition state. Our findings provide critical insights into the hydride transfer mechanism of the DHFR-catalyzed reaction, which is not only a target for antiproliferative drugs but also a paradigmatic model in mechanistic enzymology.