A molecular dynamics study on the role of the protonation state in the biosynthesis of R-PAC by AHAS
Abstract
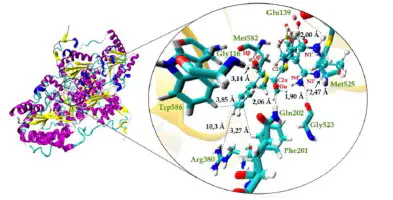
In this work, we studied the effect of the protonation state of the hydroxyl-ethylthiamin diphosphate intermediate (HEThDP) on enzyme-substrate interactions and its implications for the biosynthesis of R-phenylacetylcarbinol (R-PAC) by acetohydroxy acid synthase (AHAS) using molecular dynamics simulations. We found that the protonation state of HEThDP that favors the formation of R-PAC is the one in which the 4-aminopyrimidine ring has the N1′ atom protonated and the N4′ atom in the aminopyrimidinium ion form. In this state, both active sites of AHAS are capable of performing catalysis, unlike what is observed for other possible protonation states of the N1′ and N4′ atoms
Type
Publication
Chemical Physics Letters