On the Nature of the Enzyme - Substrate Complex and the Reaction Mechanism in Human Arginase I. A Combined Molecular Dynamics and QM/MM Study
Abstract
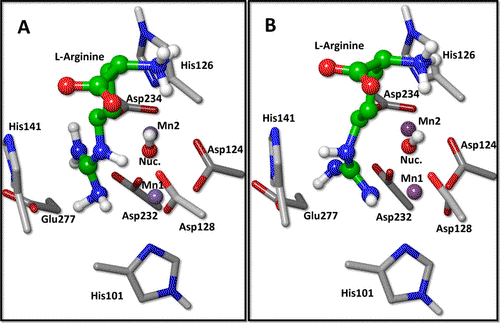
In this work, we conducted a comprehensive theoretical analysis of l-arginine hydrolysis catalyzed by human arginase I (HARGI). We combined classical molecular dynamics (MD) simulations of various enzyme-substrate complex models with a detailed exploration of the reaction mechanism using hybrid quantum mechanics/molecular mechanics (QM/MM) techniques. Different enzyme-substrate models were constructed, considering two possibilities for the nucleophile: a water molecule or a hydroxide anion bridging the two manganese(II) cations in the active site. For the substrate, we examined four scenarios: one with the guanidino group protonated and three with the group neutral, including two tautomeric states and two coordination modes with the divalent ions. Our MD simulations revealed that the most stable complexes feature a hydroxide anion as the nucleophile, while both protonated and neutral forms of the guanidino group can bind to the active site. Analysis of the potential energy surface uncovered a complex reaction pathway, where the nucleophile’s initial attack is followed by the inversion of the epsilon nitrogen atom of the guanidino group. This step produces a reaction intermediate stabilized by an H-PI interaction with a histidine residue, resembling the structure of potent HARGI inhibitors derived from 2(S)-amino-6-boronohexanoic acid. Subsequently, a proton is transferred from the nucleophile to the leaving group via Asp128, culminating in the separation of the reaction products: l-ornithine and urea. Our results are robust, supported by comparisons across different initial structures and functionals, and align well with experimental activation energy estimates and mutagenesis analysis. Finally, we demonstrated that the manganese ions play a structural and electrostatic role, stabilizing the nucleophile and intermediate states, without participating in charge-transfer processes during the reaction pathway.