Mechanistic study of the biosynthesis of R-phenylcarbinol by acetohydroxyacid synthase enzyme using hybrid quantum mechanics/molecular mechanics simulations
Abstract
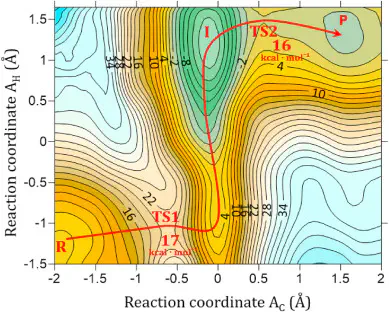
In this work, we studied the biosynthesis of R-phenylacetylcarbinol (R-PAC) by acetohydroxy acid synthase (AHAS) using molecular dynamics simulations (MD), hybrid quantum mechanics/molecular mechanics (QM/MM), and QM/MM free energy calculations. Our results show that the reaction begins with the nucleophilic attack of the C2α atom of the HEThDP intermediate on the Cβ atom of the carbonyl group of the benzaldehyde substrate, forming a transition state (TS1) with the HEThDP intermediate in the 4′-aminopyrimidinium (APH+) form. The calculated activation free energy for this step is 17.4 kcal mol⁻¹ at 27 °C. The reaction then proceeds with the abstraction of the Hβ atom of the HEThDP intermediate by the Oβ atom of benzaldehyde, forming intermediate I. The reaction concludes with the cleavage of the C2α-C2 bond, producing R-PAC and regenerating the ylide intermediate in the APH+ form, thereby restarting the catalytic cycle. The calculated activation barrier for this final step is 15.9 kcal mol⁻¹ at 27 °C.