Understanding the Interactions of Ubiquitin-Specific Protease 7 with Its Substrates through Molecular Dynamics Simulations: Insights into the Role of Its C-Terminal Domains in Substrate Recognition
Abstract
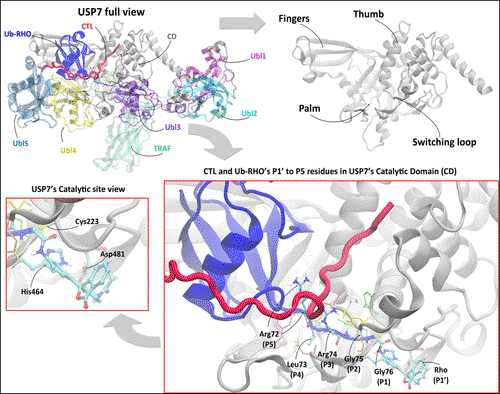
In this work, we investigated the role of the C-terminal loop (CTL) motif in ubiquitin-specific protease 7 (USP7), a deubiquitinase enzyme critical for regulating cellular processes by cleaving ubiquitin molecules from target proteins. Recent experimental evidence suggests that the CTL motif contributes to USP7’s catalytic activity by enhancing structural stability, substrate recognition, and catalytic efficiency. To elucidate these roles, we conducted extensive molecular dynamics (MD) simulations to analyze the conformational dynamics and protein - protein interactions within the USP7 enzyme - substrate complex, using a substrate composed of ubiquitin tagged with the fluorescent label rhodamine 110-gly (Ub-Rho). Our results demonstrate that the CTL motif plays a crucial role in stabilizing the conformation of the Ubl domains and increasing the prevalence of active conformations in the enzyme - substrate complex. In contrast, the absence of the CTL motif leads to increased flexibility and variability in the motion of the Ubl domains, resulting in a reduced percentage of active conformations. Additionally, our analysis of protein - protein interactions highlight the importance of the CTL motif in anchoring the Ubl45 domains to the catalytic domain (CD), thereby facilitating stable interactions with the substrate. Overall, our findings provide valuable insights into the conformational dynamics and protein - protein interactions within the USP7 enzyme - substrate complex, shedding light on the mechanistic details of substrate recognition prior to catalytic action.