Mechano-chemical coupling in biomolecular motors: The case of the Zika virus NS3-helicase
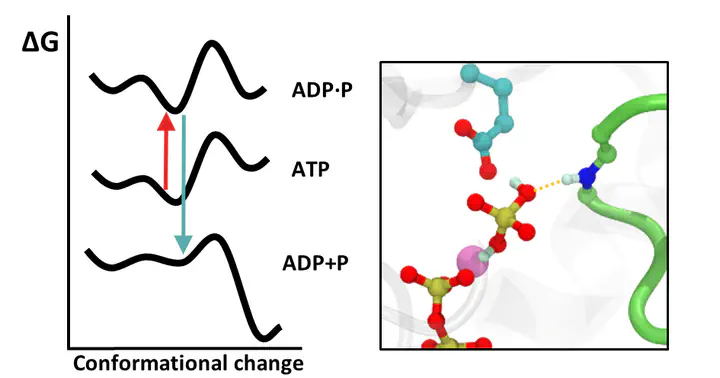
We investigate how the hydrolysis of nucleotides, such as ATP, drives conformational changes in biomolecular motors, particularly in the helicase enzyme.
Helicase plays a crucial role in viral replication by unwinding viral RNA replication complexes, a process tightly coupled to ATPase activity at its catalytic site. Understanding this mechanism at the molecular level could provide valuable insights into antiviral strategies targeting helicase function. Specifically, we focus on the Zika virus NS3-helicase and study how the different steps in the ATP cycle influence the conformational dynamics of conserved protein sequences related with helicase activity. Our first results are available in JACS. There we show how the detachment of the phosphate ion, generated after hydrolysis, from the coordination sphere of the Mg²⁺ cofactor triggers a shift in the conformational landscape of a conserved protein sequence, which ultimately leads to a change in conformation.