Chemical mechanism of N-glycosylation through molecular dynamics and experimental approaches
The project aims to unravel the chemical mechanism of N-glycosylation in oligosaccharyltransferase (OST) complex, a crucial post-translational modification that influences protein folding, stability, and function, particularly for membrane and secretory proteins.
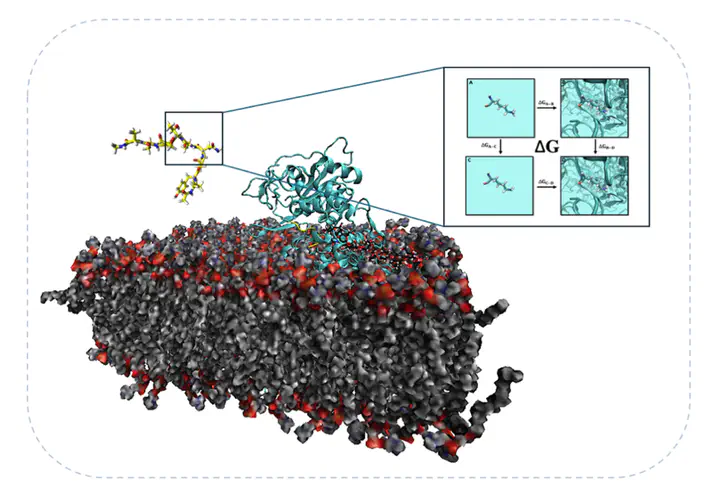
The project aims to unravel the chemical mechanism of N-glycosylation in oligosaccharyltransferase (OST) complex, a crucial post-translational modification that influences protein folding, stability, and function.
N-glycosylation is catalyzed by the oligosaccharyltransferase (OST) complex which transfers an oligosaccharide to the asparagine residue in the N-X-S/T consensus sequon. Using molecular dynamics (MD) simulations and free energy methods we investigate how mutations in specific positions in the sequon influence OST binding and catalysis catalysis and thus N-glycosylation efficiency. This work is complemented by experimental approaches, including glycosylation assays in cell cultures with a model membrane protein to validate computational predictions. These findings hold potential for optimizing glycosylation processes in many biotechnology applications. Collaborators: Prof. Ismael Mingarro Muñoz (https://www.uv.es/uvweb/universidad/es/ficha-persona-1285950309813.html?p2=mingarro&idA=).