Loop Dynamics and Reactivity of Orotidine 5'-Monophosphate Decarboxylase
This study focuses on the enzyme Orotidine 5’-Monophosphate Decarboxylase (OMPDC), which catalyzes the decarboxylation of OMP to UMP in the pyrimidine biosynthesis pathway.
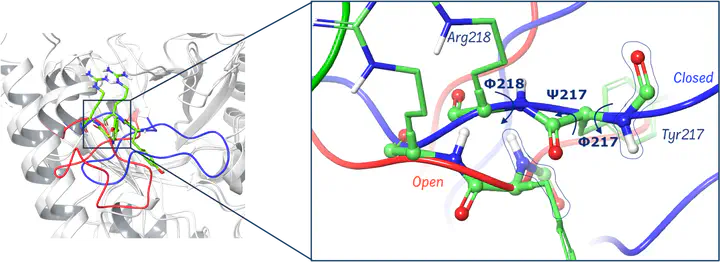
OMPDC’s catalytic efficiency relies on its flexibility, particularly involving loop 8 (Pro202-Val220), which shifts between open and closed conformations to facilitate substrate binding and catalysis. The enzyme binds the substrate flexible initially and then stiffens to stabilize the transition state, thereby lowering the activation barrier. In this study we use molecular dynamics (MD) simulations and enhanced sampling methods to investigate the conformational change of loop 8 between open and closed states. Additionally, QM/MM simulations are used to analyze the decarboxylation reaction in dependence of the loop conformation. The phosphate gripper loop (loop 8) and the pyrimidine umbrella loop play critical roles in stabilizing the substrate and shielding it from solvent. The study also addresses the presence or absence of a specific water molecule (watCO2) in the active site and its potential impact on catalysis. Ultimately, the objective is to understand how conformational changes, especially loop dynamics, are coupled to catalysis in OMPDC, providing insights useful for enzyme engineering.